Answer:
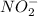 is hydrolyzed with the formation of
is hydrolyzed with the formation of
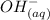 ions.
ions.
Step-by-step explanation:
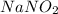 is basic because it dissolves in water to form
is basic because it dissolves in water to form
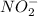 ion which further hydrolyzed in water to give
ion which further hydrolyzed in water to give
 ions.
ions.
The chemical reaction involving :
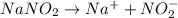
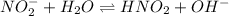
The hydroxide ions furnished in the above reaction is responsible for the
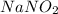 to be basic.
to be basic.