Answer:
d)2.13 C s⁻¹
Step-by-step explanation:
Rate of flow of heat through walls
=
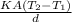
K = .33
A = 6 X .4 X .4 =0.96
T₂-T₁ = 30+40 = 70
d = 5 x 10⁻³
Put these data in the relation above
Rate of flow of heat
=
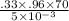
= 4435.2 Js⁻¹
Specific heat of gas = 2.5 R = 20.785 J
Rise in temp =
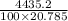
= 2.13 degree celsius.