Answer:
number of water molecules are 7
Step-by-step explanation:
Mass of the hydrated salt = 4.93 g
Mass of the dehydrated salt = 2.41 g
Mass of water lost = 4.93 - 2.41 g = 2.52 g
The moles of the dehydrated salt is :
Amount = 2.41 g
Molar mass of
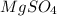 = 120 g/mol
= 120 g/mol
The formula for the calculation of moles is shown below:

Thus, moles are:
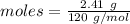
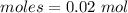
The moles of water is :
Amount = 2.52 g
Molar mass of
 = 18 g/mol
= 18 g/mol
The formula for the calculation of moles is shown below:

Thus, moles are:
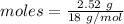
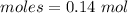
The simplest ration of the two are:
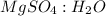 = 0.02 : 0.14 = 1 : 7
= 0.02 : 0.14 = 1 : 7
The formula is
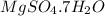
So, number of water molecules are 7