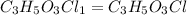Answer: The empirical formula for the given compound is
Step-by-step explanation:
The chemical equation for the combustion of compound having carbon, hydrogen, iron and oxygen follows:
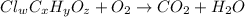
where, 'w', 'x', 'y' and 'z' are the subscripts of chlorine, carbon, hydrogen and oxygen respectively.
We are given:
Mass of
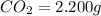
Mass of
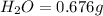
We know that:
Molar mass of carbon dioxide = 44 g/mol
Molar mass of water = 18 g/mol
For calculating the mass of carbon:
In 44 g of carbon dioxide, 12 g of carbon is contained.
So, in 2.200 g of carbon dioxide,
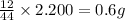 of carbon will be contained.
of carbon will be contained.
For calculating the mass of hydrogen:
In 18 g of water, 2 g of hydrogen is contained.
So, in 0.676 g of water,
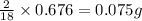 of hydrogen will be contained.
of hydrogen will be contained.
For calculating the mass of chlorine:
Now we have to calculate the moles of AgCl.
Moles of AgCl =
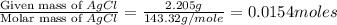
Now we have to calculate the moles of chlorine.
As we know that, 1 mole of AgCl dissociate to give 1 mole of silver ion and 1 mole of chloride ion.
So, the moles of chloride ion = Moles of AgCl = 0.0154 mole
Now we have to calculate the mass of chlorine.
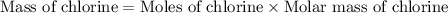
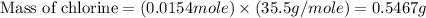
Now we have to calculate the mass percent of chlorine.
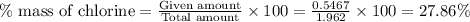
So, the amount of chlorine present in 1.962 g of compound =
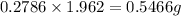
Mass of oxygen in the compound = (1.962) - (0.6 + 0.075 + 0.5466) = 0.7404 g
To formulate the empirical formula, we need to follow some steps:
Step 1: Converting the given masses into moles.
Moles of Carbon =
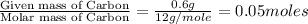
Moles of Hydrogen =
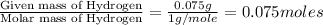
Moles of Oxygen =
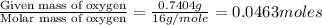
Moles of Chlorine =
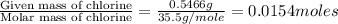
Step 2: Calculating the mole ratio of the given elements.
For the mole ratio, we divide each value of the moles by the smallest number of moles calculated which is 0.0154 moles.
For Carbon =
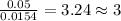
For Hydrogen =
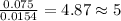
For Oxygen =
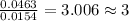
For Chlorine =
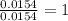
Step 3: Taking the mole ratio as their subscripts.
The ratio of Cl : C : H : O = 1 : 3 : 5 : 3
Hence, the empirical formula for the given compound is