Step-by-step explanation:
Molecular formulas is the actual number of atoms of each element in the compound while empirical formulas is the simplest or reduced ratio of the elements in the compound.
Thus,
Molecular mass = n × Empirical mass
The empirical formula is
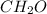
The two substances are differed by their physical properties.
These properties match with :
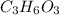 in which n =3 is molecular formula for lactic acid. Lactic acid is exists in liquid state and have a biting order.
in which n =3 is molecular formula for lactic acid. Lactic acid is exists in liquid state and have a biting order.
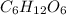 in which n =6 is molecular formula for glucose. Glucose is exists in crystalline solid form and is odorless.
in which n =6 is molecular formula for glucose. Glucose is exists in crystalline solid form and is odorless.