Answer:
Answers are given below.
Step-by-step explanation:
Balanced reaction:
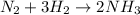
So, 3 molecules of
 react completely with 1 molecule of
react completely with 1 molecule of
 and produce 2 molecules of
and produce 2 molecules of
 .
.
Hence, 6 molecules of
 react completely with
react completely with
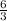 or 2 molecules of
or 2 molecules of
 and produce
and produce
 or 4 molecules of
or 4 molecules of

(A) number of
 molecules = 4
molecules = 4
(B) number of
 molecules = (6-2) =4
molecules = (6-2) =4
(C) number of
 molecules = (6-6) = 0
molecules = (6-6) = 0
Equation (D) represents best this reaction.