Answer:
Dry ice is nothing more than the solid form of
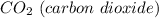 .
.
Step-by-step explanation:
It starts sublimating (goes from solid form directly to gas form) at -78.5º C, at a standard atmospheric pressure (1 atm), meaning
 can be preserved in its solid form at temperatures lower than -78.5º C.
can be preserved in its solid form at temperatures lower than -78.5º C.
Hope it helped,
BiologiaMagister