Step-by-step explanation:
As it is given that
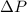 = 3 atm =
= 3 atm =
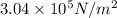
r = 73 mN/m
 =
=
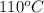
As it is known that free energy of a wetting liquid in a solid-liquid interface is lower than the solid-gas interface free energy.
As a result, there will be spontaneous filling of pores but we need a non-reactive gas under the pressure so that the liquid can be forced out of the pores.
The pressure difference will be represented as
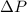 . Hence, work done by the gas to increase interface free energy is as follows.
. Hence, work done by the gas to increase interface free energy is as follows.
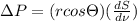
where,
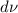 = incremental volume of gas in the pore
= incremental volume of gas in the pore
dS = incremental solid-gas interface area due to
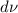 .
.
So,
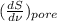 =
=
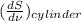
Therefore, formula to calculate pore size is as follows.
D =
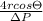
Putting the given values into the above formula as follows.
D =
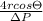
=
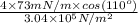
=
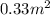
Thus, we can conclude that pore size is
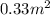 .
.