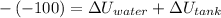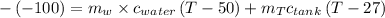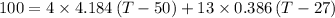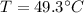Answer:49.3
Step-by-step explanation:
Given
mass of water
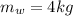
mass of tank
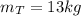
initial of temperature of water =
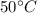
initial of temperature of tank=
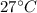
Specific heat of water =4.184kJ/kg k
Specific heat of copper=0.386 KJ/kg k
From first law of thermodynamics we have
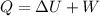
Given tank is insulated thus Q=0
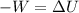
work will be negative as it is being done on system