Answer:
Ole was at loss of $0.5142.
Step-by-step explanation:
Volume of the gas at 65.3°F =
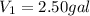
Volume of gas at 10.5°F =
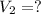
Fahrenheit to Kelvin conversion:
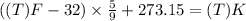
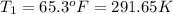
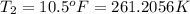
Using Charles law: Volume of gas is directly related to temperature of the gas under constant pressure.
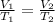
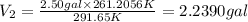
Cost of 1 gal of gas = $1.97
Cost of 2.50 gal of gas = 2.50 × $1.97 =$4.925
Cost of 2.2390 gal of gas = 2.2390 × $1.97 =$4.4108
Loss of money = $4.925 - $4.4108 = $0.5142