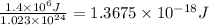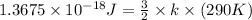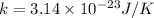Answer:
The value of of the Boltzmann constant is
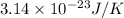 .
.
Step-by-step explanation:
Generally the formula for average kinetic energy of a molecule :
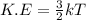
where,
k = Boltzmann’s constant
T = temperature = 290 K
Given:
Number of molecules in 1.7 moles:
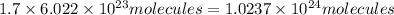
Average kinetic energy of 1.7 moles = 1.4 MJ =
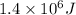
Average kinetic energy of 1 molecule =