Answer:
Lead metal has a greater magnitude of temperature change.
Step-by-step explanation:
let the mass of copper and lead be m.
Energy loosed by Copper metal = -Q = -100 J
(Negative sign just indicates that energy is released)
Change in temperature warm of the copper =

Specific heat capacity of copper = c = 0.093 KCal/Kg°C = 389.112 J/kg°C
1 kCal = 4184 Joules
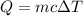
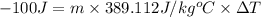
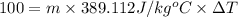
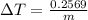
Energy gained by lead metal = Q' = 100 J
Change in temperature lead=
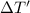
Specific heat capacity of lead = c' = 0.031 KCal/Kg°C = 129.704 J/kg°C
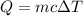
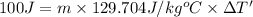
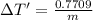
On comparing temperature changes in both metals:
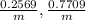
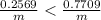

Lead metal has a greater magnitude of temperature change.