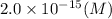Answer:
solubility of
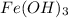 is
is
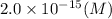
Step-by-step explanation:
Solubility equilibrium of Iron(III) hydroxide:
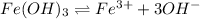
If solubility of
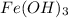 is S (M) then concentration of
is S (M) then concentration of
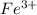 ,
,
![[Fe^(3+)]](https://img.qammunity.org/2020/formulas/chemistry/college/ds4mba1pqcw7r4lg4jsv08d34dsp5xwub7.png) is S (M)
is S (M)
We know, solubility product,
![K_(sp)=[Fe^(3+)][OH^(-)]^(3)](https://img.qammunity.org/2020/formulas/chemistry/college/f6imzjdvtvn6l3gxwkb1z6jdo06fhl67fr.png)
pH=6.50
or, pOH= 14-6.50
or, pOH = 7.50
or,
![[OH^(-)]=10^(-7.50)](https://img.qammunity.org/2020/formulas/chemistry/college/b3soj29o1syzogdg5ytfmxozts2ngsbbjf.png)
So,
![S = [Fe^(3+)]=(K_(sp))/([OH^(-)]^(3))](https://img.qammunity.org/2020/formulas/chemistry/college/lsw29tlo7gcsyj9yk3ona4fkbi0w3k3453.png) =
=
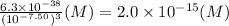
Hence solubility of
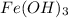 is
is