Answer:
Only option a and b follows the first law of thermodynamics.
Step-by-step explanation:
As we know that the first law of thermodynamics tells the conservation of energy,that is why it is also called quantitative law.
From the first law of thermodynamics
Q = ΔE + W
a.
Given that
W= 50 KJ,Q= 170KJ ,ΔE=120 KJ
Yes this follows the first law of thermodynamics.
b.
Given that
W= 100 Btu,Q= -110 Btu ,ΔE= -210 Btu
This also satisfy the first law of thermodynamics.
c.
Given that
W= 250 KJ,Q= -110 KJ ,ΔE=-100 KJ
Yes this does not follow the first law of thermodynamics.
d.
Given that
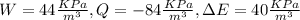
Yes this does not follow the first law of thermodynamics.
So only option a and b follows the first law of thermodynamics.