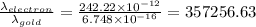Answer:
The wavelength of electron is 357256.63 times more than that of gold atom travelling at same speed.
Step-by-step explanation:
The de-broglie wavelength is obtained using the formula
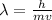
For an electron we have
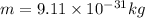
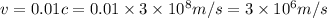
Using these values in the relation we get
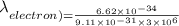
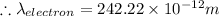
For a gold atom we have
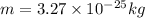
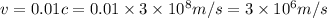
Using these values in the relation we get
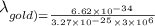
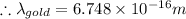
thus we can write