Step-by-step explanation:
It is given that,
Mass of boiled water, m = 4.5 kg
Radius of the pot bottom, r = 6.5 cm = 0.065 m
Thickness of the pot, t = 2 mm = 0.002 m
We need to find the temperature of the heating element in contact with the pot.
Heat transferred is from pot to water is given by :
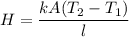
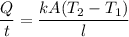
T₁ is the boiling temperature of water, T₁ = 100⁰ C
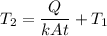 , q = m L, L is the latent heat of vaporization
, q = m L, L is the latent heat of vaporization
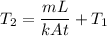 ,
,
k is the thermal conductivity of water,
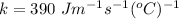
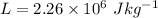
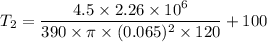
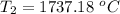
Hence, this is the required solution.