Answer:
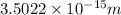 is the radius of the sodium-22 nucleus.
is the radius of the sodium-22 nucleus.
Step-by-step explanation:
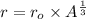
Where:
 = Constant for all nuclei
= Constant for all nuclei
r = Radius of the nucleus
A = Number of nucleons or atomic mass
Radius of the sodium-22 nucleus
Atomic mass of Na-22= 21.994434 u
A = 21.994434
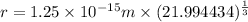

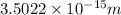 is the radius of the sodium-22 nucleus.
is the radius of the sodium-22 nucleus.