Answer:
The wavelength is 0.39 angstroms.
(A) is correct option.
Step-by-step explanation:
Given that,
Gamma ray emitted from a nucleus during radioactive decay may have an energy = 320 keV
We need to calculate the wavelength
Using formula of energy
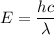
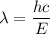
Where, E = energy
h = plank's constant
c = speed of light
Put the value into the formula
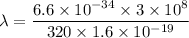
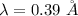
Hence, The wavelength is 0.39 angstroms.