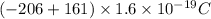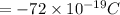Answer:
-72\times 10^{-19}C[/tex]
Step-by-step explanation:
Both electron and proton have the same amount of charge but signs are opposite , electron contains negative charge and proton contain positive charge
Charge on 1 electron =
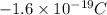
So charge on 206 electron
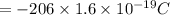
Charge on 1 proton =
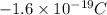
So charge on 161 electron
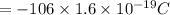
So charge of the system =