Answer:
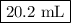
Step-by-step explanation:
Assume that the volume of the stock solution is 1 L.
1. Mass of stock solution
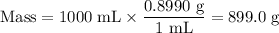
2.Mass of NH₃
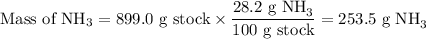
3. Moles of NH₃
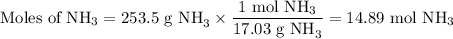
4. Molar concentration of stock solution
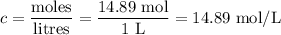
5. Volume of stock needed for dilution
Now that you know the concentration of the stock solution, you can use the dilution formula .
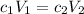
to calculate the volume of stock solution.
Data:
c₁ = 14.89 mol·L⁻¹; V₁ = ?
c₂ = 0.500 mol·L⁻¹; V₂ = 600 mL
Calculations:
