Answer:
B) 34 min.
Step-by-step explanation:
Power of oven = volt x current = 220 x 15 = 3300 Js⁻¹
Heat required to vaporize 20% of 15 kg
= mass of water x latent heat of vaporization
= 15 x .2 x 2.26 x 10⁶ = 6.78 x 10⁶
Time taken by oven to provide this heat
= heat energy / power
=
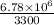
=34 min