Step-by-step explanation:
According to Le Chatelier's principle, any change in an equilibrium reaction will shift the equilibrium in the direction that will be opposing the change.
For example, in the reaction
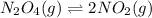 , when we increase the pressure.
, when we increase the pressure.
Then there will occur a decrease in volume of the reaction mixture. Due to this there will be increase in number of moles per unit volume.
Hence, equilibrium will shift in a direction where there are less number of moles. This means that the reaction will shift in the backward direction.
Thus, we can conclude that concentration of
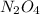 will increase as a result more dinitrogen tetraoxide will be formed.
will increase as a result more dinitrogen tetraoxide will be formed.