Step-by-step explanation:
As the given data is as follows.
Height, H = 150 feet
Heat gain = 30,000 BTU/hr, and Heat loss = 25000 BTU/hr
m = mass of water heated = 700 gallons = 5810 lbs
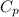 is the heat capacity of water = 1 BTU/lb
is the heat capacity of water = 1 BTU/lb
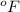 (given)
(given)
 = temperature difference =
= temperature difference =

Heat energy required to heat 700 gal can be calculated as follows:
Heat Required =
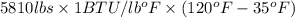
Thus, water rises till
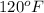 .
.