Answer:
False
Step-by-step explanation:
This statement is negates the photoelectric effect.
When an electron absorbs energy it jumps from a lower shell to a higher shell. When the electron releases energy it jumps from a higher shell to a lower shell.
The Kinetic energy of an ejected electron is
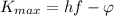
Where,
h = Planck's Constant
f = Frequency of incident photon
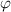 = Work function
= Work function
So, this statement is false.