Answer:
age of the site is 15411.75 years old
Step-by-step explanation:
Given data
plant or animal dies = 14C
time period = 5730 year
carbon = 15.5%
to find out
age (in years) of the ancient site
solution
we know that Final value = Initial value ×
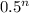
here n is half life passed
so for 15.5%
15.5% = 100% of
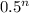
0.155 = 1 ×
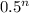
now take log both side
log 0.155 = log
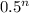
n = log 0.155 / log 0.5
n = 2.68966
we know here 5730 years in half life
so for 2.68966 half-lives = 2.68966 × 5730 = 15411.7518
age of the site is 15411.75 years old