Step-by-step explanation:
Formula to calculate heat capacity is as follows.
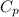 = a + bT +
= a + bT +
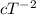
where, a, b and c are constants.
Their values at
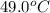 are as follows.
are as follows.
a =
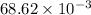
b =
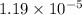
c =
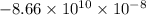
Hence, putting the given values into the above formula as follows.
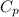 = a + bT +
= a + bT +
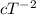
=
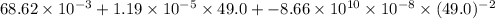 kJ/mol K
kJ/mol K
= 14.43 kJ/mol K
Thus, we can conclude that the heat capacity of crystalline calcium carbide at 49.0
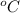 is 14.43 kJ/mol K.
is 14.43 kJ/mol K.