Answer:
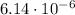
Step-by-step explanation:
Firstly, write the expression for the equilibrium constant of this reaction:
![K_(eq) = ([ADP][Pi])/(ATP)](https://img.qammunity.org/2020/formulas/chemistry/high-school/1y9kctnlnqw9lmet90fc1cvljwjnjigm9a.png)
Secondly, we may relate the change in Gibbs free energy to the equilibrium constant using the equation below:
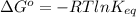
From here, rearrange the equation to solve for K:
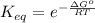
Now we know from the initial equation that:
![K_(eq) = ([ADP][Pi])/(ATP)](https://img.qammunity.org/2020/formulas/chemistry/high-school/1y9kctnlnqw9lmet90fc1cvljwjnjigm9a.png)
Let's express the ratio of ADP to ATP:
![([ADP])/([ATP]) = ([Pi])/(K_(eq))](https://img.qammunity.org/2020/formulas/chemistry/high-school/p7f7e7tw55rdhxu0rw6jfsm3acfxfc0azr.png)
Substitute the expression for K:
![([ADP])/([ATP]) = ([Pi])/(K_(eq)) = \frac{[Pi]}{e^{-(\Delta G^o)/(RT)}}](https://img.qammunity.org/2020/formulas/chemistry/high-school/jwphyfl1og7xszd2vfjebcnz14h8oddx5o.png)
Now we may use the values given to solve:
![([ADP])/([ATP]) = ([Pi])/(K_(eq)) = \frac{[Pi]}{e^{-(\Delta G^o)/(RT)}} = [Pi]e^{(\Delta G^o)/(RT)} = 1.0 M\cdot e^{(-30 kJ/mol)/(2.5 kJ/mol)} = 6.14\cdot 10^(-6)](https://img.qammunity.org/2020/formulas/chemistry/high-school/gk4ab9uxldjif6gpwgonffvtzpifu6artc.png)