Answer:
decay constant is 1.290 ×
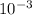 s-1
s-1
Number is 0.870 ×
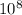 nuclei
nuclei
activity is 1.1223 ×
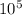 Ci
Ci
Step-by-step explanation:
Given data
isotope N1 = 6.030×10^8 nuclei
half life T/2 = 537 s
to find out
decay constant and How many nuclei remain and activity of the sample at the beginning
solution
we know that half life and decay constant relationship that is
N = N1 ×
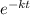 ...............1
...............1
here k is decay constant and we know dN / dt is equal to -k × N
and T/2 = 0.693/k
so k = 0.693 / (T/12)
put here T/2
k = 0.693 / 537 = 1.290 ×
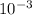 s-1
s-1
so decay constant is 1.290 ×
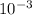 s-1
s-1
and
by equation 1 we get
N = N1 ×
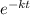
N = 6.030×10^8 ×
![e^{-1.290 × [tex]10^(-3)](https://img.qammunity.org/2020/formulas/physics/college/qs5ecjono7zqnc9cne2cpdfcf5j8vi3b6b.png) 1.50×10^3}[/tex]
1.50×10^3}[/tex]
N = 0.870 ×
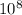 nuclei
nuclei
and
we know Activity of sample =k×N
so
activity = 0.870 ×
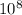 × 1.290 ×
× 1.290 ×
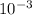
activity = 1.1223 ×
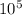 Ci
Ci