Answer:
The work done is 5136.88 J.
Step-by-step explanation:
Given that,
n = 1.90 mol
Temperature = 296 K
If the initial volume is V then the final volume will be V/3.
We need to calculate the work done
Using formula of work done
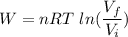
Put the value into the formula
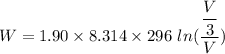
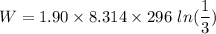
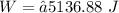
The Work done on the system.
Hence, The work done is 5136.88 J.