Answer : The value of
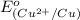 is, 0.34 V
is, 0.34 V
Explanation :
Here, copper will undergo reduction reaction will get reduced. Zinc will undergo oxidation reaction and will get oxidized.
The oxidation-reduction half cell reaction will be,
Oxidation half reaction:
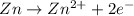
Reduction half reaction:
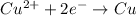
Oxidation reaction occurs at anode and reduction reaction occurs at cathode. That means, gold shows reduction and occurs at cathode and chromium shows oxidation and occurs at anode.
The overall balanced equation of the cell is,
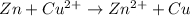
To calculate the
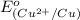 of the reaction, we use the equation:
of the reaction, we use the equation:
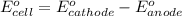
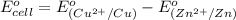
Putting values in above equation, we get:
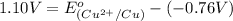
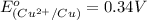
Hence, the value of
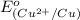 is, 0.34 V
is, 0.34 V