Answer:
0.2775 mg
Step-by-step explanation:
Electrical Energy = 345 kWh
Converting to joules
1 kWh = 1000×3600 J
345×1000×3600 = 1242×10⁶ J
Energy used for gas = 225 therm
1 therm = 29.3 kWh
So,
225 therm = 225 × 29.3 = 6592.5 kWh = 6592.5×1000×3600
Total energy = 345×1000×3600+6592.5×1000×3600 = 6937.5×1000×3600
Mass–energy equivalence
E=mc²
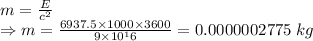
∴ Mass that would need to be converted directly to energy each month to meet the energy needs for the home is 0.2775 mg