Step-by-step explanation:
The given data is as follows.
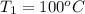 ,
,
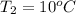
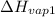 = 2257 kJ/kg,
= 2257 kJ/kg,
 = ?
= ?
For water,
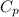 = 4.184
= 4.184
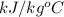
Formula to calculate heat of vaporization is as follows.
 =
=
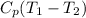
Hence, putting the values into the above formula as follows.
 =
=
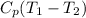
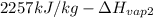 =
=
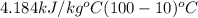
 = 2257 kJ/kg - 376.56 kJ/kg
= 2257 kJ/kg - 376.56 kJ/kg
= 1880.44 kJ/kg
Thus, we can conclude that enthalpy of liquid water at
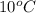 is 1880.44 kJ/kg.
is 1880.44 kJ/kg.