Answer : The sketches of electrons shared during covalent bonding are shown below.
Explanation:
Covalent bond : It is defined as the bond that is formed when sharing of electrons takes place between the atoms.
This is usually formed between two non-metals.
(a) Water
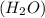
It is a covalent compound in which hydrogen and oxygen are non-metals. The water is formed when two hydrogen atoms share their electrons with oxygen atom.
(b) Carbon tetrafluoride
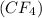
It is a covalent compound in which carbon and fluorine are non-metals. The carbon tetrafluoride is formed when four fluorine atoms share their electrons with carbon atom.
(c) Phosphorous pentafluoride
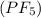
It is a covalent compound in which phosphorous and fluorine are non-metals. The phosphorous pentafluoride is formed when five fluorine atoms share their electrons with phosphorous atom.