Answer: The correct answer is Option a.
Step-by-step explanation:
- The chemical equation for warming of water from 0°C to 100°C follows:
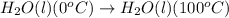
To calculate the amount of heat absorbed, we use the equation:
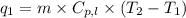
where,
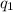 = amount of heat absorbed = ?
= amount of heat absorbed = ?
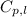 = specific heat capacity = 4.186 J/g °C
= specific heat capacity = 4.186 J/g °C
m = mass of water = 1 kg = 1000 g (Conversion factor: 1 kg = 1000 g)
 = final temperature =
= final temperature =
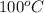
 = initial temperature =
= initial temperature =
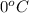
Putting all the values in above equation, we get:
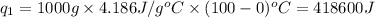
- The chemical equation for vaporization of water follows:
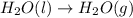
To calculate the amount of heat released, we use the equation:
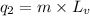
where,
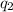 = amount of heat absorbed = ?
= amount of heat absorbed = ?
m = mass of water = 1 kg
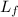 = latent heat of vaporization = 2260000 J/kg
= latent heat of vaporization = 2260000 J/kg
Putting all the values in above equation, we get:
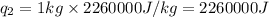
From the calculations above, we get that
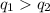
So, the warming of water requires more energy.
Hence, the correct answer is Option a.