Answer: The correct answer is Option B.
Step-by-step explanation:
The relationship of Gas constant (R) and Boltzmann constant (k) is given as:
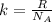 ......(1)
......(1)
We also know that:
Number of particles in a given number of moles is equal to the number of moles multiplied by Avogadro's number.
Mathematically,
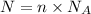
where,
N = number of particles
n = number of moles
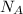 = Avogadro's number
= Avogadro's number
Putting the value of Avogadro's number in equation 1, we get:
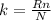
Rearranging the equation, we get:
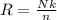
Hence, the correct answer is Option B.