Step-by-step explanation:
Normal moles of
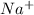 = volume × normal concentration
= volume × normal concentration
= 4.7 × 0.139 = 0.6533 mol
Moles of
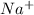 in hyponatremia blood = volume × hyponatremia concentration
in hyponatremia blood = volume × hyponatremia concentration
= 4.7 × 0.116 = 0.5452 mol
Moles of NaCl to be added = moles of extra
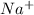 needed
needed
= 0.6533 mol - 0.5452 mol = 0.1081 mol
Mass of NaCl = moles × molar mass of NaCl
= 0.1081 mol × 58.443
= 6.317g
= 6.32 g (approx)
Thus, we can conclude that mass of sodium chloride would need to be added to the blood is 6.32 g.