Answer:
K(maximum) = 0.284 eV
Step-by-step explanation:
Given data
work function ω = 3.60 eV = 3.60 × 1.6 ×
 = 5.76 ×
= 5.76 ×
 J
J
wavelength λ = 320 nm = 320 ×
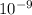 m
m
to find out
maximum kinetic energy
solution
we know that speed of light is = 3 ×
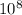 m/s
m/s
and planck constant h = 6.63 ×
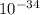 J.s
J.s
we will apply here Einstein's photo elecric equation that is
hc/λ = ω + K(maximum)
put here all these value we get
6.63 ×
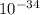 × 3 ×
× 3 ×
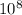 / 320 ×
/ 320 ×
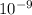 = 5.76 ×
= 5.76 ×
 + K(maximum)
+ K(maximum)
so
K(maximum) = 6.63 ×
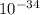 × 3 ×
× 3 ×
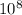 / 320 ×
/ 320 ×
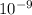 - 5.76 ×
- 5.76 ×

K(maximum) = 0.4556 ×
 J
J
K(maximum) = 0.4556 ×
 / 1.6 ×
/ 1.6 ×

K(maximum) = 0.284 eV