Answer:
601 nm
Step-by-step explanation:
Energy of photon having wavelength of λ nm
=
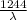 eV
eV
Energy of 249 nm photon
=
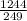
=4.996 eV
Similarly energy of 425nm photon
=
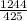
=2.927 eV
Difference = 2.069 eV.
This energy will give rise to another photon whose wavelength will be
λ =
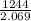
= 601 nm.