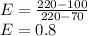Answer:
Oil has the smaller heat capacity. The effectiveness of the heat exchanger is 0.80.
Step-by-step explanation:
Part 1:
In order to know which fluid has the smaller heat capacity we need to consider the heat equation below:
Q = CΔT, where Q is the heat exchanged, C is the heat capacity and ΔT is the variation in temperature.
As the heat exchange is the same for both fluids, the smaller the temperature variation, the smaller the heat capacity.
Water: ΔT = 120 °F
Oil: ΔT = 80 °F
Therefore, oil is the fluid with the smallest heat capacity.
Part 2:
The effectiveness of a counter-flow heat exchanger is given by the equation bellow:
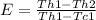
Th1: initial temperature of the hot fluid
Th2: final temperature of the hot
Tc1: initial temperature of the cold fluid