Answer:
For a: The entropy change is positive.
For b: The entropy change is negative.
For c: The entropy change is positive.
For d: The entropy change is positive.
Step-by-step explanation:
Entropy change is defined as the change in the measure of randomness in the reaction. It is represented as
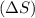 . Randomness of gaseous particles is more than that of liquid which is further more than that of solids.
. Randomness of gaseous particles is more than that of liquid which is further more than that of solids.
In a chemical reaction, if the solid reactant is getting converted to liquid or gas or even aqueous state (ions are getting formed), then the entropy change is positive.
If the case is reverse, then the entropy change is taken as negative.
For the given options:
Option a:
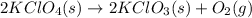
As, the solid reactant is getting converted into gaseous product. Thus, the randomness will increase and hence, the entropy change is positive.
Option b:
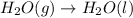
As, the gaseous reactant is getting converted into liquid product. Thus, the randomness will decrease and hence, the entropy change is negative.
Option c:
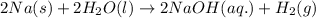
As, the solid and liquid reactants are getting converted into gaseous and aqueous products. Thus, the randomness will increase and hence, the entropy change is positive.
Option d:
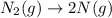
As, nitrogen molecule is getting converted to two nitrogen atoms. So, the number of atoms are getting increases which increases the randomness of the system. Hence, the entropy change is positive.