Answer:
wavelength is 121.58 nm
Step-by-step explanation:
given data
wavelength = 230 nm = 230 x
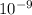 m
m
kinetic energy = 3.35 ×
 J
J
to find out
wavelength of light that triple the maximum kinetic energy of the electron
solution
we know here Einstein's photo electric equation that is given below
hc / λ = φ + K(maximum)
and here φ will be
φ = (hc) / λ - K(max imum)
put here h = 6.626 x
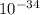 J.s
J.s
and 3.0 x
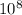 m/s
m/s
so φ = (6.626 x
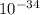 )( 3.0 x
)( 3.0 x
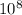 ) / ( 230 x
) / ( 230 x
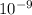 ) - 3.35 x
) - 3.35 x

φ = 6.2995 x
 J
J
so here we have given triple the maximum kinetic energy
so
hc / λ = φ + K(maximum)
λ = hc / ( φ + K(maximum) )
λ = (6.626 x
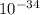 )( 3.0 x
)( 3.0 x
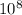 ) / ( 6.2995 x
) / ( 6.2995 x
 + 3 ( 3.35 x
+ 3 ( 3.35 x
 ))
))
λ = 1.215817 ×
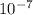
so wavelength is 121.58 nm