Answer : The concentration of
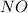 at equilibrium is 0.9332 M
at equilibrium is 0.9332 M
Solution : Given,
Concentration of
 and
and
 at equilibrium = 0.200 M
at equilibrium = 0.200 M
Concentration of
 and
and
 at equilibrium = 0.500 M
at equilibrium = 0.500 M
First we have to calculate the value of equilibrium constant.
The given equilibrium reaction is,
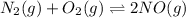
The expression of
 will be,
will be,
![K_c=([NO]^2)/([N_2][O_2])](https://img.qammunity.org/2020/formulas/chemistry/college/h97e3v9ia7fb0kvxd3enqoq711mg7wqk69.png)
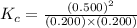

Now we have to calculate the final concentration of NO.
The given equilibrium reaction is,
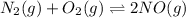
Initially 0.200 0.200 0
.800
At equilibrium (0.200-x) (0.200-x) (0.800+2x)
The expression of
 will be,
will be,
![K_c=([NO]^2)/([N_2][O_2])](https://img.qammunity.org/2020/formulas/chemistry/college/h97e3v9ia7fb0kvxd3enqoq711mg7wqk69.png)
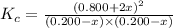
By solving the term x, we get
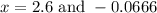
From the values of 'x' we conclude that, x = 2.6 can not more than initial concentration. So, the value of 'x' which is equal to 2.6 is not consider.
And the negative value of 'x' shows that the equilibrium shifts towards the left side (reactants side).
Thus, the concentration of
 at equilibrium = (0.800+2x) = 0.800 + 2(0.0666) = 0.9332 M
at equilibrium = (0.800+2x) = 0.800 + 2(0.0666) = 0.9332 M
Therefore, the concentration of
 at equilibrium is 0.9332 M
at equilibrium is 0.9332 M