Step-by-step explanation:
Let the age to be found in years is y.
Hence,
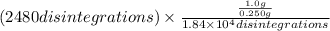
= (\frac{1}{2})^{\frac{y}{5730yr}}
Solve for y as follows.
0.53913 = (\frac{1}{2})^{\frac{y}{5730yr}}
Now, taking log on both the sides as follows
log 0.53913 =
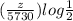
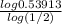 =
=
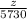
z =
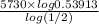
= 5107 years
Thus, we can conclude that the time since death is 5107 years.