Answer: The molar solubility of MX is

Step-by-step explanation:
We are given:
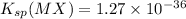
The chemical equation for the ionization of MX follows:

S S
The expression of
 for above equation is:
for above equation is:
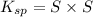 ......(1)
......(1)
The chemical equation for the ionization of
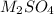 follows:
follows:
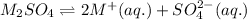
0.25M 0.5M 0.25M
Total concentration of cation from both the equation is:
![[M^+]=0.5+S](https://img.qammunity.org/2020/formulas/chemistry/college/y52zyklcswnk08mgex4w3399rqmq5bq5ah.png)
As,
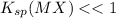 , so S is also very very less than 1 and can be easily neglected.
, so S is also very very less than 1 and can be easily neglected.
So,
![[M^+]=0.5M](https://img.qammunity.org/2020/formulas/chemistry/college/t4xta75f0hirnfl6wue1v0gaombsq9wfog.png)
Putting values in equation 1, we get:

Hence, the molar solubility of MX is
