Answer:
0.05110 mol/L is the concentration of water at equilibrium.
Step-by-step explanation:
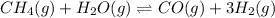
An equilibrium constant of the given reaction =

Volume of the container = V = 0.64 L
Concentration =
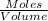
Concentration of CO=
![[CO]=(0.36 mol)/(0.64 L)](https://img.qammunity.org/2020/formulas/chemistry/college/v9hk4rp91je64lgp8d0mg8ibxp2rfnfsxe.png)
Concentration of
![H_2=[H_2]=(0.081 mol)/(0.64 L)](https://img.qammunity.org/2020/formulas/chemistry/college/wf3e60w861s7fw6h8ih991v4c6odzighfh.png)
Concentration of
![CH_4=[CH_4]=(0.051 mol)/(0.64 L)](https://img.qammunity.org/2020/formulas/chemistry/college/8dgcej5877m18w0dzbd6xwnc4bm8nw93s8.png)
Concentration of
![H_2O=[H_2O]=?](https://img.qammunity.org/2020/formulas/chemistry/college/1yujd905jn85mp8upcswqohl19ho1agnla.png)
An expression of an equilibrium constant is given as:
![K_c=([CO][H_2]^3)/([CH_4][H_2O])](https://img.qammunity.org/2020/formulas/chemistry/college/q0xv20eho44vqhfrbvhyj5i6ka78bq02vr.png)
![0.28=((0.36 mol)/(0.64 L)* ((0.081 mol)/(0.64 L))^3)/((0.051 mol)/(0.64 L)* [H_2O])](https://img.qammunity.org/2020/formulas/chemistry/college/1dfnbguc23r629nnvbwmgo8qlr1tfo53va.png)
![[H_2O]=0.05110 mol/L](https://img.qammunity.org/2020/formulas/chemistry/college/b58jmwghr2ca24xc8hwmfjrsjet8ocsnk9.png)
0.05110 mol/L is the concentration of water at equilibrium.