Answer:
The mass of coke needed to react completely with 1.0 ton of copper(II) oxide is 0.794 Ton.
Step-by-step explanation:
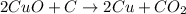
1 Ton = 907185 grams
Mass of copper oxide = 1.0 Ton = 907185 grams
Moles of copper oxide =
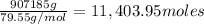
According to reaction, 2 moles of copper oxide reacts with 1 mole of carbon.
Then 11403.95 moles of copper oxide will react with:
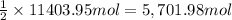 of carbon
of carbon
Mass of 5,701.98 moles of carbon:
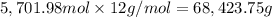
Mass of coke = x
Mass of carbon = 68,423.75 g
Percentage of carbon in coke = 95%
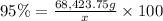
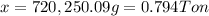
The mass of coke needed to react completely with 1.0 ton of copper(II) oxide is 0.794 Ton.