Answer:
1 .
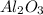
2.
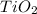
Step-by-step explanation:
The more stable the ionic compound, the more is it lattice energy.
- The more the charge on the cation and the anion, the greater is the lattice energy.
- The less the size of the cation and the anion, the greater is the lattice energy.
Scandium oxide (
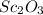 ) is an oxide in which
) is an oxide in which
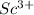 behaves as cation and
behaves as cation and
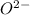 behaves as anion.
behaves as anion.
The compounds which has higher lattice energy than scandium oxide are:
1 .
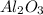
This is because the charge are same on the cation and the anion as in the case of the Scandium oxide but the size of the cation
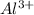 is smaller than
is smaller than
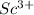 . Thus, this corresponds to higher lattice energy.
. Thus, this corresponds to higher lattice energy.
2.
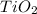
This is because the charge on the cation
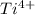 is greater than that of
is greater than that of
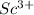 and also the size of the cation
and also the size of the cation
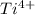 is smaller than
is smaller than
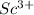 . Thus, this corresponds to higher lattice energy.
. Thus, this corresponds to higher lattice energy.