Answer :
(a) The limiting reactant is,

(b) The mass of
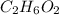 is, 11570 grams
is, 11570 grams
(c) The mass of
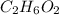 is, 5785 grams
is, 5785 grams
Explanation : Given,
Molar rate of
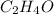 = 80 kg/s = 80000 g/s
= 80 kg/s = 80000 g/s
Molar rate of
 = 25.5 lbm/s = 11.57 kg/s = 11570 g/s
= 25.5 lbm/s = 11.57 kg/s = 11570 g/s
conversion used : (1 lbm = 0.453592 kg)
Molar mass of
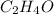 = 44 g/mole
= 44 g/mole
Molar mass of
 = 18 g/mole
= 18 g/mole
Molar mass of
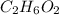 = 50 g/mole
= 50 g/mole
First we have to calculate the moles of
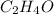 and
and
 per second.
per second.
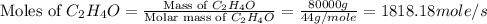
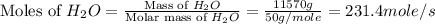
Now we have to calculate the limiting and excess reagent.
The balanced chemical reaction is,
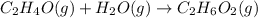
From the balanced reaction we conclude that
The mole ratio of
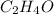 and
and
 is, 1 : 1
is, 1 : 1
From this we conclude that,
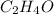 is an excess reagent because the given moles are greater than the required moles and
is an excess reagent because the given moles are greater than the required moles and
 is a limiting reagent and it limits the formation of product.
is a limiting reagent and it limits the formation of product.
Now we have to calculate the moles of
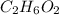 .
.
As, 1 moles of
 react to give 6 moles of
react to give 6 moles of
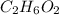
So, 231.4 moles of
 react to give 231.4 moles of
react to give 231.4 moles of
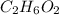
Now we have to calculate the mass of
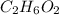 .
.
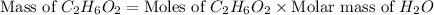
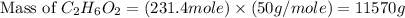
Now we have to calculate the mass of of
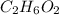 .
.
As we are given that
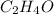 is 50 % pure.
is 50 % pure.
So, the mass of
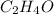 =
=
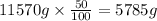
The mass of
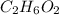 is, 5785 grams
is, 5785 grams