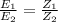Answer:
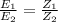
Step-by-step explanation:
Let us assume W1 g of a substance and W2 g of another substance are produced at two different electrodes when same quantity of electricity is passed through them.
if two elements have their respective electro-chemical equivalents Z1 and Z2 and their corresponding chemical equivalents are E1 and E2.
Then according to Faraday's first law,
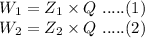
Divide eq.(1) by eq.(2)
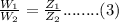
and according to Faraday's second law,
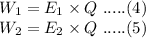
Divide eq.(4) by eq.(5)
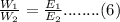
From equation (3) and (6) we get,