Step-by-step explanation:
Electronic configuration is defined as the representation of total number of electrons that are present in an atom around the nucleus.
Valence electrons are defined as the electrons which are present in the outermost shell of the atom.
For the given options:
The number of electrons present in sodium atom is 11.
Electronic configuration of Na (Z = 11):
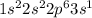
Number of valence electrons = 1
The number of electrons present in bromine atom is 35.
Electronic configuration of Br (Z = 35):
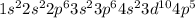
Number of valence electrons = 7
The number of electrons present in oxygen atom is 8.
Electronic configuration of O (Z = 8):
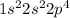
Number of valence electrons = 6
The number of electrons present in sulfur atom is 16.
Electronic configuration of S (Z = 16):
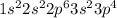
Number of valence electrons = 6
The number of electrons present in silver atom is 47.
Electronic configuration of Ag (Z = 47):
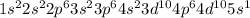
Number of valence electrons = 1